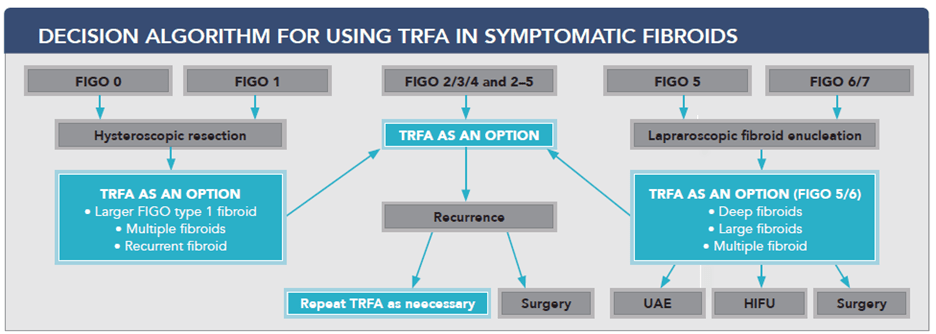
The Combined Clinical Experience of Over 1000 Sonata Procedures, Across Three Countries, Drives New Recommendation for Sonata as a First Line Therapy for Fibroid Treatment
Redwood City, CA, August 11, 2021 – Gynesonics®, a women’s healthcare company focused on the development of minimally invasive solutions for symptomatic uterine fibroids, announced today a group of 12 surgeons from multiple fibroid treatment centers in Germany, Austria and Switzerland have published a review of symptomatic fibroid treatments, and recommended a treatment algorithm based on fibroid type and location.1 In this algorithm, Gynesonics’ Sonata® System was placed as the primary treatment procedure for 4 of 9 fibroids types (FIGO types 2, 3, 4 and 2-5) encompassing over 60% of fibroid occurrence. In the remaining applicable fibroids (FIGO types 1, 5 and 6), the recommendation was that Sonata should be considered for certain common scenarios such as larger and deeper fibroids that may be more difficult to treat surgically, including staged treatments or for women that have multiple fibroids. This supports utilizing Sonata as a treatment for more than 80% of fibroid types. This recommendation came shortly before The American College of Obstetricians and Gynecologists (ACOG) published new guidelines also supporting the use of Transcervical Radiofrequency Ablation (TRFA) for the treatment of symptomatic fibroids. Sonata is the only commercially available TRFA system. This expert consensus led to a new recommended treatment algorithm for each fibroid type:
“When we began evaluating transcervical radiofrequency fibroid ablation (TRFA) using Sonata, we quickly learned that this technique, a completely incision free procedure, would be applicable for a wide range of fibroid types and patients,” said Prof. Dr. med Thomas Römer, Evangelisches Klinikum Köln-Weyertal, in Cologne, Germany. “This creates the opportunity for many more women to take advantage of the extremely fast recovery and durable results. This procedure can be placed in the operating theatre workflow quickly and in a straightforward manner.”
The Sonata technology platform integrates the first and only commercial intrauterine ultrasound system with a proprietary advanced radiofrequency ablation device, providing an incision-free, uterus-preserving, transcervical treatment for symptomatic uterine fibroids. The Sonata Treatment is a breakthrough alternative to hysterectomy and myomectomy, and can treat a wider range of fibroid types, sizes, and locations than hysteroscopic myomectomy. Fibroids are treated from within the uterus, so treatment with the Sonata System requires no incisions, no tissue is surgically removed, and the uterus is retained.
“This new fibroid treatment algorithm will be useful for physicians in the United States who are now evaluating where Sonata should be placed in their clinical protocols,” said Kelly Roy, MD, Arizona Gynecology Consultants, Assistant Program Director FMIGS Banner University Medical Center and Clinical Assistant Professor, University of Arizona College of Medicine-Phoenix. “This is an unprecedented opportunity to build on the clinical experience of these surgeons, who have performed over 1000 Sonata procedures in multiple, established fibroid treatment centers. This published experience will allow physicians to create a protocol more rapidly and confidently, including patient selection criteria, utilizing Sonata (TRFA). This review is aligned with the recent practice guidance by ACOG that recognizes the role of transcervical fibroid ablation as an important option for women in the treatment of symptomatic fibroids.2 Additionally, the Sonata System allows the surgeon to treat the most common types of myomas, which are not accessible by any other transcervical method, and therefore Sonata is the only fibroid ablation system noted in the ACOG guidelines that can avoid abdominal incisions.”
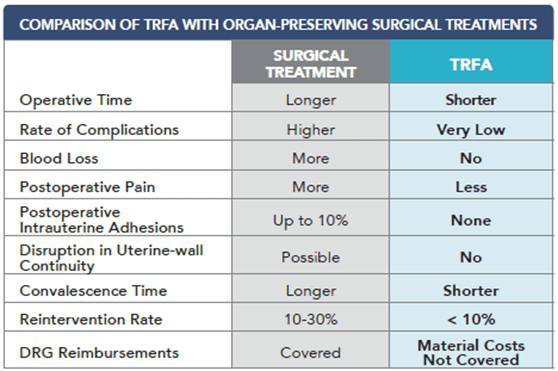
After reviewing published clinical results, including procedure time, recovery time, complication rate and reintervention rate, the authors found the TRFA procedure to be favorable in the majority of characteristics.
For more information about the Sonata System, see https://gynesonics.com/us/sonata-system/.
References
- Römer T, Bends R, Christoffel L et al. Behandlung von symptomatischen Myomen mit der transzervikalen ultraschallgesteuerten Radiofrequenzablation-Indikationen, Durchführung, Ergebnisse und Komplikationen-Expertenkonsensus 2020. Teil 2: Die transzervikale Radiofrequenzablation (TRFA) – Methode, Indikationen, Ergebnisse und Vergleich mit anderen Therapien. Frauenarzt 2021;62:162-168. (English translation at gynesonics.com)
- Management of Symptomatic Uterine Leiomyomas: ACOG Practice Bulletin, Number 228. Obstet Gynecol. 2021;1131-1133. acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas.
About Sonata System
The Sonata System uses radiofrequency energy to ablate fibroids under real time sonography guidance from within the uterine cavity, utilizing the first and only intrauterine ultrasound transducer. The system includes a proprietary graphical user interface (SMART Guide), enabling the operator to target fibroids and optimize treatment. The Sonata System provides incision-free transcervical access for a uterus-preserving fibroid treatment. This intrauterine approach is designed to avoid the peritoneal cavity. Most side effects are typically minor and temporary. The Sonata System is CE marked and is approved for sale in the European Union, the United Kingdom, and the United States.
About Fibroids1,2
Fibroids are benign growths in or around the uterus. They are common and most women develop them during childbearing age. In the U.S., around 70% of white women and more than 80% of black women will have uterine fibroids before the age of 50. Problematic fibroids vary in size, and symptoms may worsen over time if fibroids are left untreated. 20% to 50% of women with fibroids are symptomatic and each year in the U.S., more than 2 million women undergo treatment for uterine fibroids. Women with symptomatic fibroids may present with one or more of the following: abnormal uterine bleeding/menorrhagia, abdominopelvic pain/pressure, increased abdominal girth, urinary frequency, constipation, subfertility, pregnancy complications, dyspareunia (painful intercourse).
- Baird DD, Dunson DB, Hill MC et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;100-107.
- Stovall DW. Clinical Symptomatology of Uterine Leiomyomas. Clinical Obstetrics and Gynecology. 2001;364-371.
About the FIGO (International Federation of Obstetrics and Gynecology) Fibroid Subclassification1,2
The International Federation of Gynecology and Obstetrics (FIGO) has developed a fibroid subclassification to guide clinicians and investigators in the treatment of abnormal uterine bleeding due to leiomyomata (fibroids) (AUB-L). This system includes the categorization of submucous, intramural and subserous fibroids. In this subclassification, type 0, type 1, and type 2 fibroids represent submucous fibroids and, as they indent or lie within the endometrial cavity, are strongly associated with heavy menstrual bleeding. Type 3 and type 4 fibroids lie within the muscle layer of the uterus (myometrium) and are thus intramural. Intramural fibroids are the most common fibroid type and are believed to cause abnormal uterine bleeding, and if large (typically >4-5 cm), may also cause “bulk” symptoms like pressure and painful intercourse (dyspareunia). Type 5, type 6 and type 7 fibroids are those that abut or extend beyond the outside portion of the uterus (serosa). These have no established effects on menstrual blood loss but if large, are associated with bulk symptomatology. Hybrid FIGO type 2-5 fibroids are transmural, in that they occupy the width of the uterine wall, and as they indent the endometrial cavity, are strongly associated with heavy menstrual bleeding and can also cause bulk symptoms when large.
- Munro MG, Critchley HO, Broder MS et al. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;3-13.
- Munro MG, Critchley HOD, Fraser IS, FIGO MDC. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018;393-408.
About Gynesonics
Gynesonics is a women’s healthcare company focused on advancing women’s health, by developing minimally invasive, incision-free, uterus-preserving, transcervical technologies for diagnostic and therapeutic applications. Gynesonics has developed the Sonata System for diagnostic intrauterine imaging and transcervical treatment of symptomatic uterine fibroids. Gynesonics headquarters is in Redwood City, CA. For more information, go to www.gynesonics.com.
# # #
Company Contact:
Stan Van Gent, Senior Director, Global Marketing, Gynesonics, svangent@gynesonics.com, (661) 388-6380
Media Contact:
David Gutierrez, Dresner Corporate Services, dgutierrez@dresnerco.com, (312) 780-7204